Academic Publications
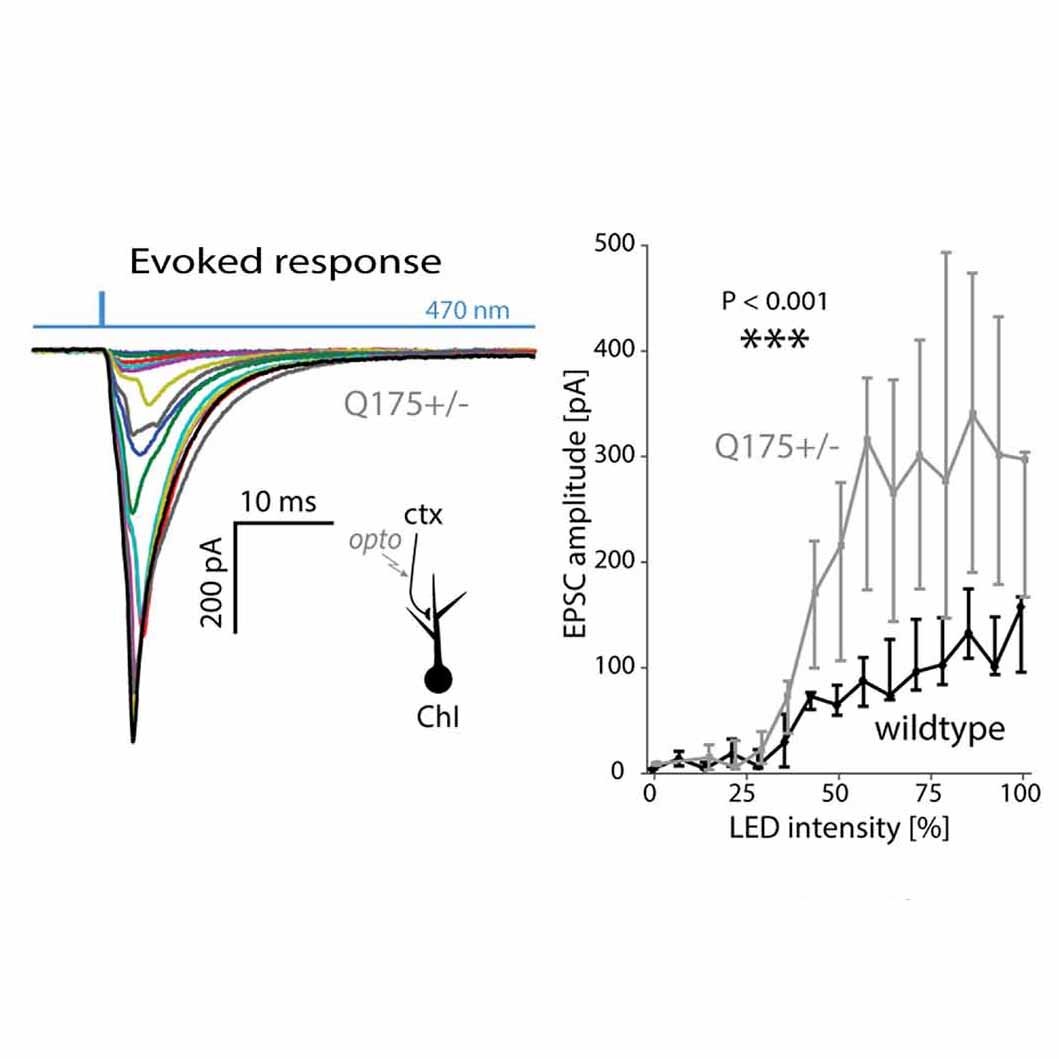
My primary interest in neurobiology is to understand the circuitry of a brain region called the striatum. The striatum is important for everyday functions such as motor learning, decision making, and motivation. Improper neuronal signaling in the striatum is related to a wide variety of disorders, including Parkinson's disease, addiction, schizophrenia, and OCD. Getting a clearer picture of the way parts of the brain communicate with the striatum will help us develop therapies for these disorders.
I use electrophysiological techniques in combination with optogenetics and pharmacology to understand the cells in the striatum. The majority of my research uses patch-clamp electrophysiology (whole cell, cell attached, and perforated patch) in an ex vivo slice preparation. Using this technique, I am able to study the way striatal cells communicate.